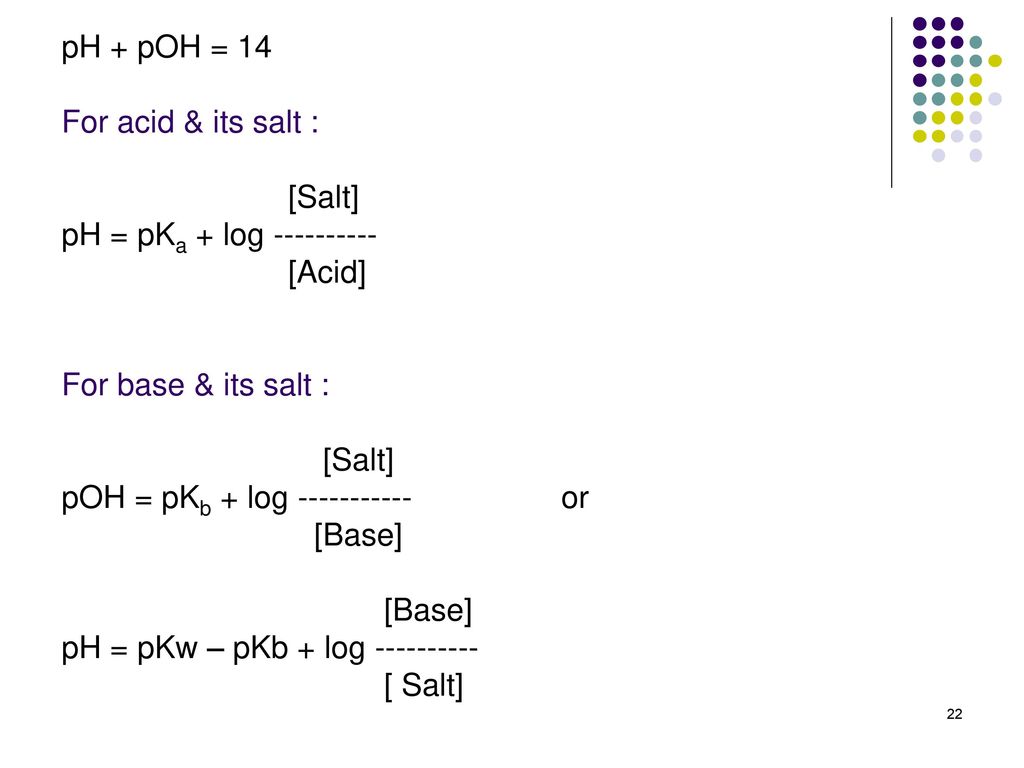
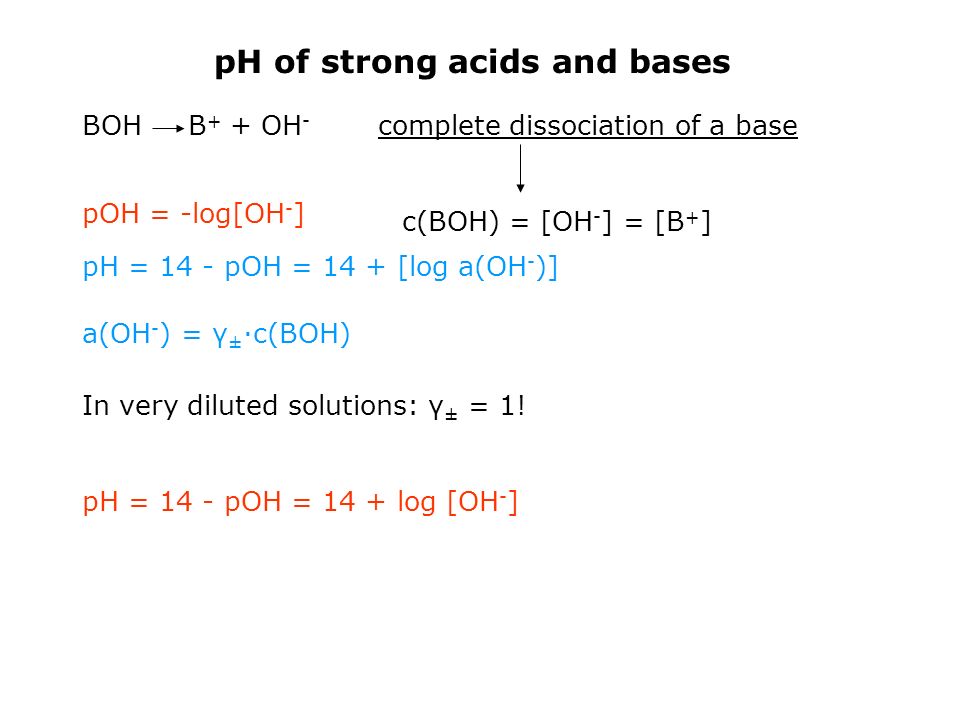
Questions on chapter 8 - HL 18.0 Lewis theory 1 - Describe what the Lewis theory is. 18.1 Calculations involving acids and bases 2 - How is Kw defined ? 3 - How are pH, pOH and pKw defined ? 4 - What is the relationship between pH, pOH and pKw ? 5 ...
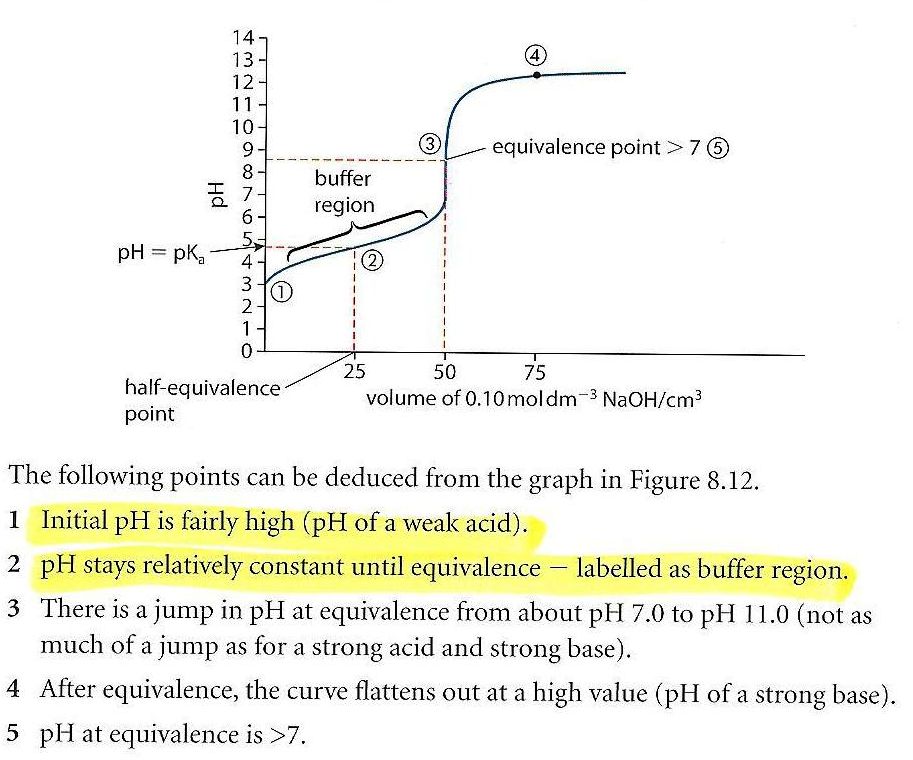
Questions on chapter 8 - HL 18.0 Lewis theory 1 - Describe what the Lewis theory is. 18.1 Calculations involving acids and bases 2 - How is Kw defined ? 3 - How are pH, pOH and pKw defined ? 4 - What is the relationship between pH, pOH and pKw ? 5 ...
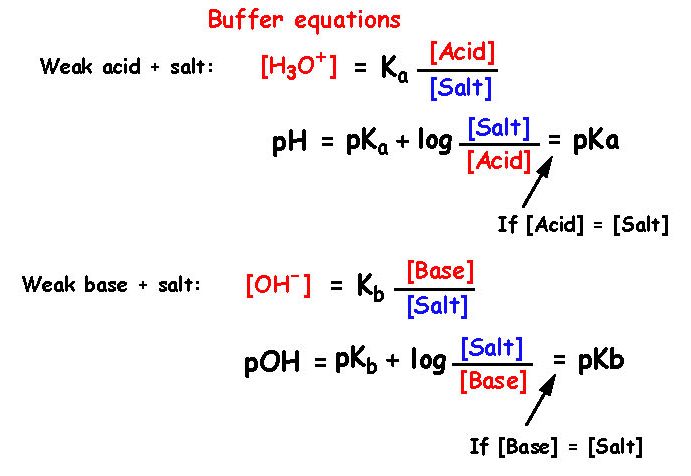
Questions on chapter 8 - HL 18.0 Lewis theory 1 - Describe what the Lewis theory is. 18.1 Calculations involving acids and bases 2 - How is Kw defined ? 3 - How are pH, pOH and pKw defined ? 4 - What is the relationship between pH, pOH and pKw ? 5 ...
![Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube](https://i.ytimg.com/vi/SLPu7qlUdEA/maxresdefault.jpg)
Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube
![OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo... OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/119/11951925.png)









/ph-water-measurement-157442701-570e9e295f9b58140891668e.jpg)